Anthracyclines remain a cornerstone of therapy for many hematologic malignancies, owing to their potent cytotoxic effects on rapidly proliferating cancer cells. However, their use is limited by well-recognized cardiotoxicity, which can manifest across a broad temporal spectrum and significantly affect long-term morbidity and mortality among cancer survivors.
Patterns and Incidence of Cardiotoxicity
Anthracycline-associated cardiotoxicity is classically described as acute, early-onset, or late-onset.
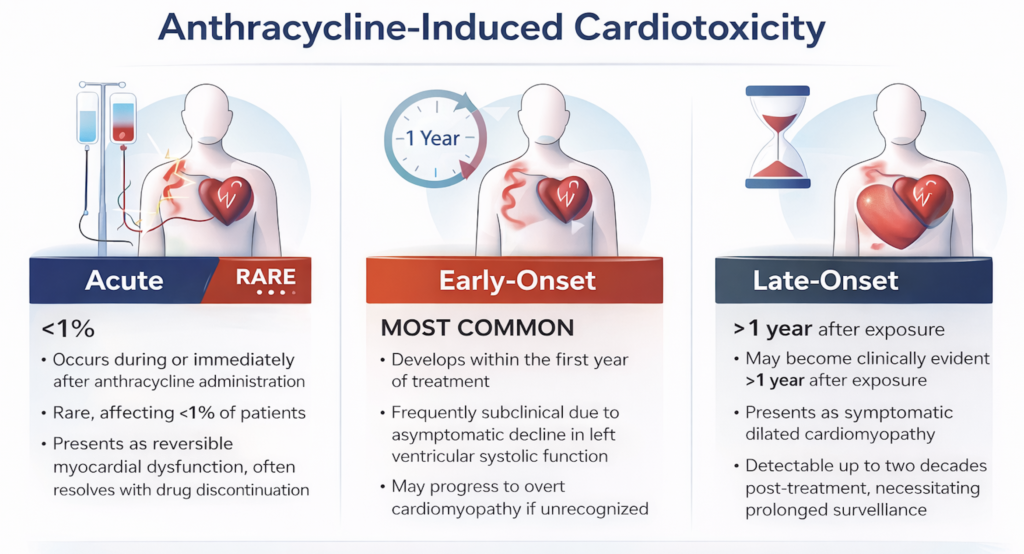
Acute cardiotoxicity occurs during or immediately after anthracycline administration and is rare, affecting fewer than 1% of patients. It typically presents as reversible myocardial dysfunction, and often resolves with drug discontinuation.
Early-onset cardiotoxicity, which develops within the first year of treatment, represents the most common manifestation. It is frequently subclinical and characterized by an asymptomatic decline in left ventricular systolic function. Without timely recognition, this stage may progress to overt cardiomyopathy.
Late-onset cardiotoxicity may become clinically evident more than one year after exposure and often presents as symptomatic dilated cardiomyopathy. Importantly, long-term observational studies have demonstrated that anthracycline-related cardiotoxicity can remain detectable for up to two decades following treatment, underscoring the need for prolonged surveillance (1).
In a large meta-analysis of 22,815 patients exposed to anthracyclines, the overall incidence of clinically apparent cardiotoxicity was approximately 6%, while 18% demonstrated subclinical cardiac dysfunction (2). With the incorporation of advanced imaging modalities such as cardiac magnetic resonance imaging and speckle-tracking echocardiography with global longitudinal strain (GLS), the prevalence of subclinical cardiotoxicity has been estimated to be as high as 40% (3).
Pathophysiology
Anthracycline-induced cardiotoxicity is multifactorial and incompletely understood. Key mechanisms include:
- Oxidative stress, leading to mitochondrial and sarcoplasmic reticulum injury, resulting in impaired energy metabolism and calcium homeostasis
- Disruption of cardiomyocyte cell death pathways, promoting apoptosis and necrosis
- Emerging mechanisms, including epigenetic modifications and altered gene expression
Genetic susceptibility is an area of active investigation. Variants associated with non-ischemic cardiomyopathies (e.g., titin mutations), genes involved in reactive oxygen species production and detoxification, and pathways related to anthracycline metabolism may modulate individual risk.
Myocardial injury often begins as asymptomatic left ventricular dysfunction and may progress to irreversible heart failure if not recognized early. Experimental and animal studies suggest the existence of a dose threshold below which cardiomyocyte injury may be partially reversible, emphasizing the importance of cumulative dose awareness.
Dose Dependency and Modifying Factors
Anthracyclines are incompletely metabolized within cardiomyocytes, and continued exposure leads to intracellular accumulation. For doxorubicin, the traditionally recognized cumulative dose threshold associated with increased cardiotoxicity risk is approximately 250–300 mg/m².
However, this threshold is not absolute. Concurrent cancer therapies, particularly chest radiation, along with preexisting cardiovascular risk factors such as hypertension, diabetes, dyslipidemia, and advanced age, can accelerate myocardial injury and precipitate cardiac events at substantially lower cumulative doses in high-risk populations.
Hematologic Malignancies: Therapeutic Context
In hematologic malignancies, the high proliferative rate of malignant cells confers substantial sensitivity to anthracyclines, making them integral to many curative regimens. Over time, clinical research has supported strategies aimed at dose reduction, substitution with pegylated liposomal formulations, and anthracycline-sparing regimens when feasible.
Despite these advances, anthracyclines continue to play a critical role in the treatment of many blood cancers, necessitating careful cardiovascular risk stratification and surveillance.
Strategies to Reduce Cardiotoxicity
Several approaches have been explored to mitigate anthracycline-related cardiotoxicity:
- Dexrazoxane, an iron-chelating agent that reduces oxidative stress, has demonstrated cardioprotective effects. Its use, however, remains limited due to concerns regarding potential interference with anthracycline efficacy and historical associations with secondary malignancies.
- Liposomal anthracycline formulations have larger particle sizes and limited myocardial diffusion, resulting in reduced cardiomyocyte exposure and lower rates of cardiotoxicity.
- Prolonged or continuous infusions lower peak plasma anthracycline concentrations, allowing enhanced systemic metabolism and reduced myocardial accumulation.
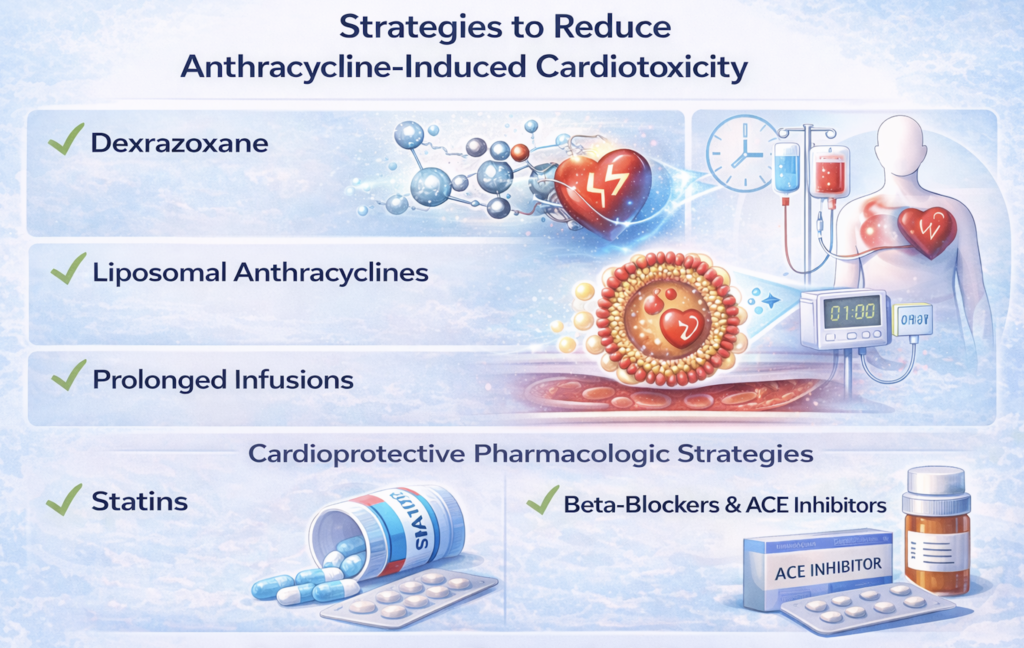
Cardioprotective Pharmacologic Strategies
Evidence supporting routine prophylactic cardioprotective therapy remains evolving. Select studies suggest that:
- Statin therapy (e.g., atorvastatin 40 mg daily) may reduce the incidence of cardiac dysfunction in high-risk lymphoma cohorts
- Beta-blockers and ACE inhibitors have shown benefit in reducing anthracycline-related cardiotoxicity in high risk breast cancer populations
However, these findings have not yet translated into universal guideline-mandated prophylaxis, and patient selection remains critical.
Conclusion
Anthracycline-associated cardiotoxicity represents a significant and enduring complication of therapy for hematologic malignancies. Its manifestations range from early, reversible myocardial dysfunction to late-onset, progressive cardiomyopathy decades after treatment. Recognition of cumulative dose effects, modifying risk factors, and advances in surveillance and cardioprotective strategies are essential to improving long-term cardiovascular outcomes in cancer survivors.
References
- Larsen CM, Garcia Arango M, Dasari H, et al. Association of anthracycline exposure with heart failure in patients treated for breast cancer or lymphoma, 1985–2010. JAMA Netw Open. 2023;6(2):e2254669.
- Lotrionte M, Biondi-Zoccai G, Abbate A, et al. Incidence and clinical predictors of anthracycline cardiotoxicity: a systematic review and meta-analysis. Am J Cardiol. 2013;112(12):1980–1984.
- Oikonomou EK, Kokkinidis DG, Kampaktsis PN, et al. Global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. 2019;4(10):1007–1018.
— Anusha Bhat, MD, MPH
Cardio-Oncology | Cardiovascular Disease
